Brow Henna Tattooing In California Approved?
Safety and Regulatory Information
FDA has received reports of adverse reactions to some "decal," henna, and "black henna" temporary tattoos. Hither is data nearly the safety of these products and how they are regulated.
- "Decal" Temporary Tattoos
- Henna, or Mehndi, and "Black Henna"
- Finding out What's in a Temporary Tattoo or Henna/Mehndi Product
- FDA'south Authority over Color Additives in Cosmetics
- FDA's Dominance over Other Cosmetic Ingredients
- FDA Enforcement Activity
- How to Written report a Reaction to a Temporary Tattoo or Other Cosmetic
- More Resources on Temporary Tattoos:
- Related Resources:
"Decal" Temporary Tattoos
Decal temporary tattoos are used to decorate any part of the trunk, including areas of the face and around the eyes, and may concluding for a day or up to a week or more than. They are peculiarly popular with children and at Halloween.
There are two kinds of decal tattoos:
- Some are images attached to a removable backing. The decal image is removed from the backing by wetting, and the image is then applied directly to the skin.
- Others have a backing that adheres to the pare, creating a partial or complete barrier between the skin and the dyes used in the prototype.
The difference is important, because non all dyes are known to be safe for utilize on the skin. While an agglutinative bankroll may protect the pare from unapproved colors, there may be other ingredients on or in the decal to aid the prototype adhere better either to the bankroll or to the skin that may crusade bug for some people.
FDA has received reports of reactions to some decal-blazon temporary tattoos. Earlier using a temporary tattoo on your face, it may be a good thought to effort it on a less conspicuous part of your trunk.
Henna, or Mehndi, and "Black Henna"
Henna, a coloring made from a plant, is approved only for use as a pilus dye. It is not approved for direct application to the skin, as in the body-decorating process known as mehndi. This unapproved employ of a color additive makes these products adulterated. It is unlawful, for case, to introduce an adulterated corrective into interstate commerce.
Because henna typically produces a brown, orange-brown, or carmine-brown tint, other ingredients must be added to produce other colors, such every bit those marketed as "black henna" and "blue henna." Even brownish shades of products marketed equally henna may contain other ingredients intended to make them darker or make the stain concluding longer on the skin.
The extra ingredient used to blacken henna is oftentimes a coal-tar hair dye containing p-phenylenediamine (PPD), an ingredient that can cause unsafe peel reactions in some people. That's the reason hair dyes have a circumspection argument and instructions to exercise a "patch test" on a small surface area of the skin before using them. Sometimes, the artist may use a PPD-containing hair dye alone. Either way, there'southward no telling who will be afflicted. By law, PPD is not permitted in cosmetics intended to exist practical to the peel.
FDA has received reports of injuries to the skin from products marketed as henna and products marketed equally "black henna." For more than information on Henna, run across the consumer update: Temporary Tattoos May Put Yous at Take chances.
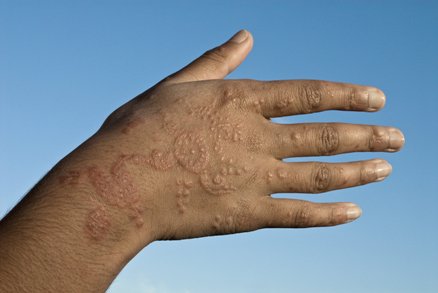
Allergic reaction on a man'due south hand. J. Cole/Photo Researchers.
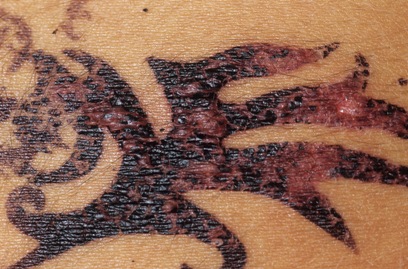
Allergic reaction on a 14-twelvemonth-old girl. Dr. P. Marazzi/Photo Researchers.
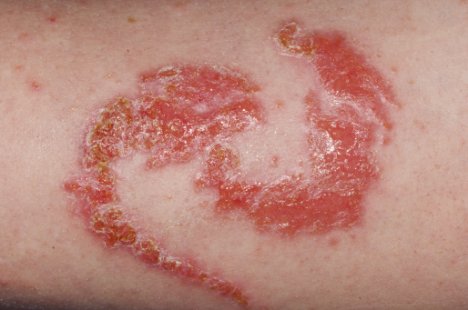
Allergic reaction on an arm. Dr. P. Marazzi/Photograph Researchers.
Finding out What's in a Temporary Tattoo or Henna/Mehndi Product
Cosmetics that are sold on a retail basis to consumers must have their ingredients listed on the label. Without such an ingredient proclamation, they are considered misbranded, and therefore it is unlawful to introduce them into interstate commerce. FDA requires the ingredient declaration nether the say-so of the Fair Packaging and Labeling Act (FPLA).
Because the FPLA does not apply to cosmetic samples and products used only by professionals--for example, for awarding at a salon, or a booth at a fair or boardwalk--the requirement for an ingredient annunciation does non employ to these products.
FDA'southward Authority over Colour Additives in Cosmetics
By law, all colour additives used in cosmetics must be approved past FDA for their intended uses, with the exception of coal tar colors intended for use in hair dyes. In addition, some colour additives must not be used unless FDA has certified that the batch meets the regulatory requirements for composition and purity. Cosmetics, including temporary tattoo products, that do non comply with restrictions on color additives are considered adulterated, and it is unlawful to innovate them into interstate commerce. To larn more, meet Colour Additives and Cosmetics, and, for information on how color additives are approved, Color Additive Petitions.
FDA's Authority over Other Cosmetic Ingredients
Cosmetics must be safe when consumers use them following directions on the label, or in the customary or expected style. Except for colour additives, the constabulary does not require cosmetic products and ingredients to have FDA approval before they are marketed. In addition, firms are not required to report their prophylactic information, including complaints.
For a list of ingredients that are prohibited or restricted in cosmetics, see "Prohibited and Restricted Ingredients."
For a list of color additives allowed in cosmetics, how they are allowed to be used, and links to their regulations, run into "Color Additives Permitted for Utilise in Cosmetics."
FDA Enforcement Action
FDA can accept action against cosmetics on the market that don't comply with the law. For example, we can issue Import Alerts and Warning Messages.
An Import Warning allows FDA to detain products that violate or appear to violate the Federal Nutrient, Drug, and Corrective Act. Nosotros accept two Import Alerts in upshot for temporary tattoos. Even so, because not all shipments of imported cosmetics are inspected, it'southward even so possible for some dangerous or mislabeled products to exist imported into this country.
- An Import Alert is in effect for several foreign-made decal temporary tattoos because they contain colors not permitted for apply in cosmetics practical to the skin, or they don't take the required ingredient list on the label, or they are labeled as "FDA canonical."
- An Import Alert is in upshot for henna intended for utilize on the pare considering it is an unapproved utilise of the color additive.
FDA issues Warning Letters to permit companies know that they take violated the constabulary and to tell them what corrective activeness they need to take. We have issued a Warning Letter to a visitor marketing "blackness henna" products:
- Warning Letters to Industry on Cosmetic-Related Issues
It is important to note that the practice of tattooing is generally regulated past country and/or local officials, and that the FDA is not typically involved in such enforcement. While states have jurisdiction over professional practices such as tattooing and cosmetology, that oversight differs from land to state. Some states accept laws and regulations for temporary tattooing, while others don't. And so, depending on where you are, it's possible no one is checking to make certain the creative person is following safety practices or even knows what may be harmful to consumers.
How to Report a Reaction to a Temporary Tattoo or Other Cosmetic
It's of import for consumers and wellness care providers to report problems with cosmetics to FDA. This data helps FDA discover out which products are causing bug, and what kinds of issues.
You tin can written report a problem with a corrective to FDA in either of these means:
- Contact MedWatch, FDA's trouble-reporting programme, on the Web or at one-800-332-1088
, or file a MedWatch Voluntary report online.
- Contact the consumer complaint coordinator in your expanse.
To larn more, see Adverse Event Reporting.
Related Resources
- Consumer Update: Temporary Tattoos May Put Yous at Risk
- Temporary Tattoos: Raising Consumer Sensation of Safety: FDA Webinar, May 13, 2014
- Warning Letter Issued to Black Henna Ink, Inc.
- Import Alert #53-19: Detention Without Concrete Examination of Henna Based Skin Color
- Lucky xiii Tips for a Safe Halloween
- Novelty Makeup: Special for Halloween
- Tattoos and Permanent Makeup
- FDA Authority Over Cosmetics
- FDA Recall Policy for Cosmetics
- Tattoos & Permanent Makeup: Quick Guide (PDF: 536 KB)
- Los Tatuajes y el Maquillaje Permanente: Una Guía (PDF: 522KB)
Source: https://www.fda.gov/cosmetics/cosmetic-products/temporary-tattoos-hennamehndi-and-black-henna-fact-sheet
Posted by: johnsonmanis1967.blogspot.com

0 Response to "Brow Henna Tattooing In California Approved?"
Post a Comment